Welcome back! Last week, we studied a rare disease called Ledderhose Disease. Many of you contacted me and gave me ideas for future blogs based on our “rare diseases” series. Thank you so much for your input, we listened! If you missed last week’s blog and would like to catch up, click HERE.

This week, we are researching a rare disease called Neurofibromatosis. Neurofibromatosis is a genetic disorder of the nervous system. It can affect how nerve cells form and grow. It causes tumors to grow on nerves. You can get neurofibromatosis from your parents, or it can happen because of a mutation (change) in your genes. Once you have it, you can pass it along to your children.
Since this writer was unfamiliar with this disease in its entirety, I relied on the following sources for information: Madeline Zupan, NORD Editorial Intern from the University of Notre Dame, Kristina Bundra, Pharm. D, NORD Editorial Intern, and Justin T. Jordan, MD, MPH, Associate Clinical Director, Pappas Center for Neuro-Oncology, Family Center for Neurofibromatosis, Massachusetts General Hospital; Instructor of Neurology, Harvard Medical School, via the NORD web site. (NORD=National Organization of Rare Disorders).
At the end of this blog, I will post an extensive list of supportive services, as well as links for investigative studies for those affected by this disease.
First of all, let’s talk about all the names this disease is known by.
- NF1
- Von Recklinghausen’s disease
- Von Recklinghausen’s neurofibromatosis
- segmental neurofibromatosis
Neurofibromatosis 1 (NF1), also called von Recklinghausen’s disease, is a genetic disorder characterized by the development of multiple noncancerous (benign) tumors of nerves and skin (neurofibromas) and areas of abnormal skin color (pigmentation).
Diagnosis:
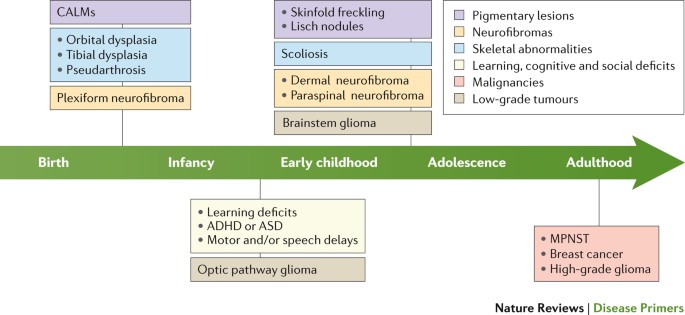
The diagnosis of NF1 is usually made during the first decade of life, based on characteristic skin freckling, cafe-au-lait spots, optic glioma, and/or pseudoarthrosis. NF1 should be suspected if any one of the criteria are present and diagnosed if two or more of the following criteria are present:
- Six or more cafe-au-lait spots 1.5 centimeters (cm) or larger in post-pubertal individuals, 0.5 cm or larger in pre-pubertal individuals
2. Two or more neurofibromas of any type or one or more “plexiform” neurofibroma
3. Freckling under the arms (axillary) or in the groin area (inguinal)
4. Non-malignant tumor of the optic nerve (optic glioma)
5. Two or more Lisch nodules in the iris of the eyes (iris hamartomas)
6. Specific bone lesions including abnormal development of the sphenoid bone of the skull (sphenoid dysplasia) or thinning, fractures, and bowing of long bones (pseudoarthrosis)
7. A parent, sibling, or child (first degree relative) with NF1
Diagnosis of NF1 is usually based on clinical findings. Laboratory tests are available to diagnose individuals where NF1 may be suspected. Some of the skin findings (i.e., café-au-lait spots) are not always easily visible and may require use of an ultraviolet light to identify. Molecular genetic testing for mutations in the NF1 gene can be used for diagnosis prior to the development of symptoms in many cases.
Symptoms:
Multiple noncancerous (benign) tumors (neurofibromas) develop in NF1 along the linings of the nerves (sheath) under the skin or in deeper areas of the body. Neurofibromas may form in any organ in the body.
Skin (cutaneous) neurofibromas, or less discrete neurofibromas (plexiform neurofibromas) may cause disfigurement. Occasionally, tumors may develop in the brain, on the nerves exiting the brain, and/or on the spinal cord. The total number of neurofibromas in an adult may range from a few to hundreds or even thousands, and the number of these tumors tends to increase with age.
Pain may occur from an affected peripheral nerve, or as a result of regional mass effect on adjacent structures. In 8-15% of affected individuals, neurofibromas may transform to become cancerous (malignant peripheral nerve sheath tumors), which are associated with pain, weight loss, night sweats, and require urgent evaluation and treatment.
Approximately 15% of people with NF1 develop brain tumors (gliomas), which nearly always develop during childhood. These frequently develop on the nerves of the eye (optic gliomas), and may affect vision or potentially lead to blindness. Additionally, a variety of other tumors may develop in patients with NF1, including gastrointestinal stromal tumors (GIST).
In women with NF1, there is a 3.5-fold increased risk of developing breast cancer and a five-fold increased risk of developing breast cancer before the age of 50 years of age.
Orthopedic problems may develop with NF1, including curvature of the spine (scoliosis), abnormal cranial bone growth (sphenoid wing dysplasia), or a condition characterized by loss of bone tissue, fractures, and abnormal healing and bowing of weight-bearing long bones (pseudoarthrosis). Additionally, disorders of bone density (osteopenia and osteoporosis) are more common in people with NF1 than in the general population.
The process by which these conditions develop is not fully understood, but has been associated with decreased activated vitamin D levels, increased parathyroid hormone levels, and increased markers of bone breakdown. People with NF1 tend to be below average in height, below average in muscle strength, and above average in head size for age.

Treatment Options:
The first treatment for NF1 was approved by the U.S Food and Drug Administration (FDA) in April 2020 to treat pediatric patients age 2 and older. Koselugo (selumetinib) is a kinase inhibitor, meaning it functions by blocking a key enzyme, which results in helping to stop the tumor cells from growing.
Another option is to undergo surgery to remove particularly troublesome or disfiguring tumors, depending on their size and location.
Laser or electrocautery treatment is also an option for smaller skin neurofibromas. Radiation therapy, chemotherapy, or both treatments may be used by some clinicians to treat NF1-associated tumors, though their role is less clear and depends on tumor type and location.
Physical therapy may be beneficial for some people. A variety of orthopedic devices may help to improve mobility in some cases. Other treatments of choice are symptomatic and supportive. For example, in patients who develop scoliosis, a brace may be necessary to prevent progression.
Neurofibromatosis 2
A related disorder is Neurofibromatosis 2 (NF2). This is a rare disorder that is genetically distinct from NF1. NF2 is characterized by benign tumors on both auditory nerves (vestibular schwannomas) and in other areas of the body. Symptoms of NF2 may include problems with balance, buzzing or ringing in the ears (tinnitus), progressive hearing loss, or facial weakness.
A note from Linda Tate:
I would like to end this blog by saying that I am committed to bringing rare diseases and disorders to light via this platform. Once we know that these diseases exist, then we can find the money to fund research that may bring an end to each of them, one disease at a time. I applaud NORD and The Children’s Tumor Foundation (Ending NF Through Research) for their ongoing commitment to this end.
Here are resources I promised at the beginning of this blog, as provided by NORD:
Investigational Therapies
Information on current clinical trials is posted on the Internet at www.clinicaltrials.gov . All studies receiving U.S. government funding, and some supported by private industry, are posted on this government website.
For more information about clinical trials being conducted at the NIH Clinical Center in Bethesda, MD, contact the NIH Patient Recruitment Office:
Tollfree: (800) 411-1222
TTY: (866) 411-1010
Email: prpl@cc.nih.gov
Some current clinical trials also are posted on the following page on the NORD website:
https://rarediseases.org/for-patients-and-families/information-resources/info-clinical-trials-and-research-studies/
For information about clinical trials sponsored by private sources, contact: www.centerwatch.com
For information about clinical trials conducted in Europe, contact: https://www.clinicaltrialsregister.eu/
The Children’s Tumor Foundation launched a Neurofibromatosis (NF) Registry in 2012. The purpose of this registry is to find people who may be eligible for clinical trials or other research studies being conducted in the field of NF, and to determine the commonality of specific NF characteristics. Please go to www.nfregistry.org for more information.
The Children’s Tumor Foundation supports the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) initiative in an effort to develop new standardized response criteria for determining treatment response with neurofibromatosis 1, neurofibromatosis 2, and Schwannomatosis. The purpose of this effort is to better compare treatment efficacy in clinical trials.
For more information about this collaboration contact: Dr. Scott R. Plotkin at splotkin@reinscollaboration.org.
Resources
RareConnect offers a safe patient-hosted online community for patients and caregivers affected by this rare disease. For more information, visit www.rareconnect.org.
NORD Member Organizations
- Children’s Tumor Foundation
- 370 Lexington Avenue
- Suite 2100
- New York, NY 10017
- Phone: (212) 344-6633
- Email: info@ctf.org
- Website: http://www.ctf.org/
- Neurofibromatosis Network
- 213 S. Wheaton Ave.
- Wheaton, IL 60187 USA
- Phone: (630) 510-1115
- Toll-free: (800) 942-6825
- Email: admin@nfnetwork.org
- Website: http://www.nfnetwork.org
- Neurofibromatosis Northeast
- 9 Bedford Street
- Burlington, MA 01803 USA
- Email: info@nfincne.org
- Website: http://nfnortheast.org/
- NORD’s™ Rare Cancer Coalition (RCC)
- 1779 Massachusetts Avenue NW
- Ste 500
- Washington, DC 20036 USA
- Phone: (202) 545-3971
- Email: Membership@RareDiseases.org
- Website: https://rarediseases.org/get-involved/rare-cancer-coalition/
- RASopathies Network USA
- 244 Taos Road
- Altadena, CA 91001-3953
- Phone: (626) 676-7694
- Email: info@rasopathiesnet.org
- Website: http://www.rasopathiesnet.org
Other Organizations
- BC Neurofibromatosis Foundation
- Box 5339
- Victoria, BC, V8R 6S4 Canada
- Toll-free: (800) 385-2263
- Email: info@bcnf.bc.ca
- Website: http://www.bcnf.bc.ca
- Cancer.Net
- American Society of Clinical Oncology
- 2318 Mill Road Suite 800
- Alexandria, VA 22314
- Phone: (571) 483-1780
- Toll-free: (888) 651-3038
- Email: contactus@cancer.net
- Website: http://www.cancer.net/
- Children’s Hospital of Philadelphia Neurofibromatosis Program
- The Children’s Hospital of Philadelphia
- 3401 Civic Center Blvd
- Philadelphia, PA 19104
- Phone: (215) 590-7012
- Toll-free: (800) 879-2467
- Website: http://www.chop.edu/centers-programs/neurofibromatosis-program#.V6i7G1UrIdU
- Genetic and Rare Diseases (GARD) Information Center
- PO Box 8126
- Gaithersburg, MD 20898-8126
- Phone: (301) 251-4925
- Toll-free: (888) 205-2311
- Website: http://rarediseases.info.nih.gov/GARD/
- Let Them Hear Foundation
- 1900 University Avenue, Suite 101
- East Palo Alto, CA 94303
- Phone: (650) 462-3174
- Email: info@letthemhear.org
- Website: http://www.letthemhear.org
- Massachusetts General Hospital Neurofibromatosis Clinic
- 15 Parkman St. 8th Floor, Room 835
- Boston, MA 2114 USA
- Phone: (617) 724-7856
- Email: SPlotkin@partners.org
- Website: http://neurosurgery.mgh.harvard.edu/NFclinic/
- Medical Home Portal
- Dept. of Pediatrics
- University of Utah
- Salt Lake City, UT 84158
- Phone: (801) 587-9978
- Email: mindy.tueller@utah.edu
- Website: http://www.medicalhomeportal.org
- Neurofibromatosis Clinic at Texas Children’s Hospital
- 6701 Fannin Street, #1560
- Houston, TX 77030
- Phone: (832) 822-4280
- Toll-free: (800) 364-5437
- Email: plunkett@bcm.edu
- Website: http://www.texaschildrens.org/departments/neurofibromatosis-clinic
- NIH/National Institute of Neurological Disorders and Stroke
- P.O. Box 5801
- Bethesda, MD 20824
- Phone: (301) 496-5751
- Toll-free: (800) 352-9424
- Website: http://www.ninds.nih.gov/
- Rare Cancer Alliance
- 1649 North Pacana Way
- Green Valley, AZ 85614 USA
- Website: http://www.rare-cancer.org
If you found this blog to be helpful, informative, or just a relaxing way to waste time, please do me a favor and share it on your Facebook page? To share, just click on the Facebook icon at the top of this page. Much appreciated!
1 Comment